
Is C2H2 Polar Or Nonpolar? Techiescientist
Hey Guys!In this video, we are going to determine the polarity of Ethane having a chemical formula of C2H6. To know the polarity of this molecule we will fir.

Is C2H6 Polar or Nonpolar? Techiescientist
C2H2 is nonpolar in nature because the electronegativity difference between Carbon and Hydrogen is 0.35, which is less than the minimum required 0.4. Furthermore, it has a linear molecular structure and the nature of C-H bonds is non-polar covalent. This makes the complete C2H2 molecule a non-polar molecule, with a net zero dipole moment.
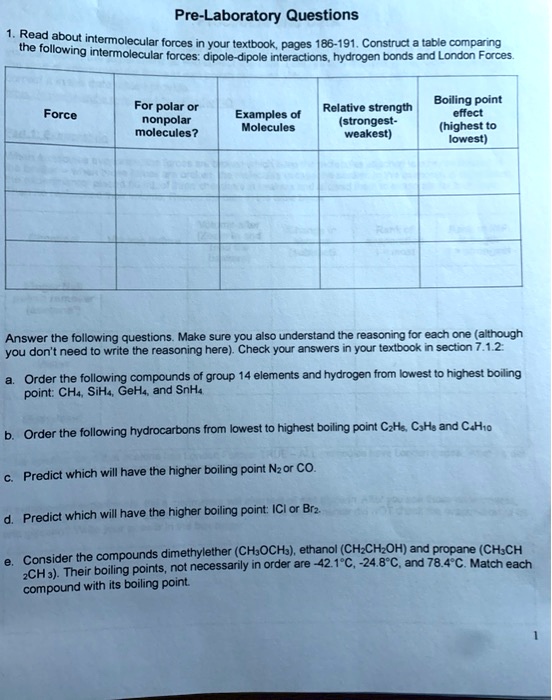
SOLVED PreLaboratory Questions Read about intermolecular forces in
C 2 H 6 is an extremely flammable compound and is a potential asphyxiation (deprivation of oxygen to the body) hazard. If you have a query regarding the polarity of the ethane (C2H6) molecule and want to know whether it is polar or non-polar, you are in the right place. Let's get started. Is C2H6 polar or non-polar?

Which of the following compounds shows an abnormal boiling point
The difference in electronegativities of atoms The shape of the molecule Dipole moment Distribution of charges C2H2 is made up of two types of atoms: Carbon and Hydrogen. The Carbon atom has an electronegativity of 2.55 and Hydrogen has an electronegativity value of 2.20

Is C2H5OH Polar or Nonpolar? (Ethanol) Polar, Molecules, Functional group
Is C2H6 Polar or Nonpolar? Answer: C2H6 (ethane) is a nonpolar molecule because it contains only nonpolar covalent bonds (C-H) bonds with both parts of the molecule cancelling out any small charge to ensure that there is no dipole moment.
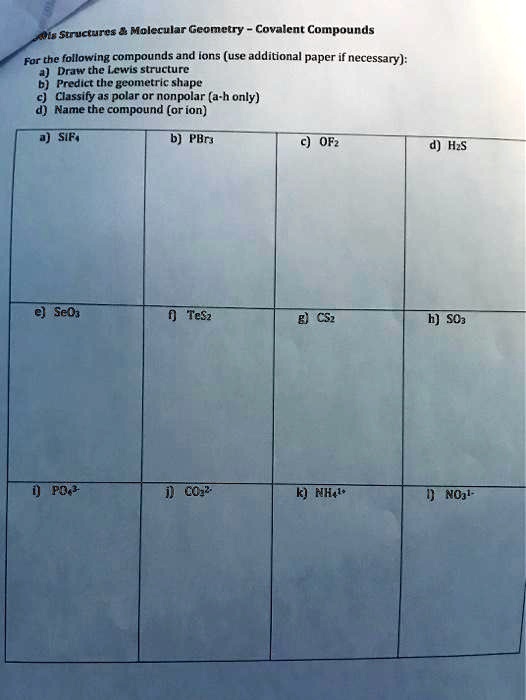
SOLVED Structures Molecular Geometry of Covalent Compounds For the
Molecular Polarity. To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons.Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms.

C2h6 Lewis Structure Polar Or Nonpolar Draw Easy
A nonpolar covalent bond is formed between same atoms or atoms with very similar electronegativities—the difference in electronegativity between bonded atoms is less than 0.5. A polar covalent bond is formed when atoms of slightly different electronegativities share electrons.

(PDF) A Framework with Nonpolar Pore Surfaces for the One
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.
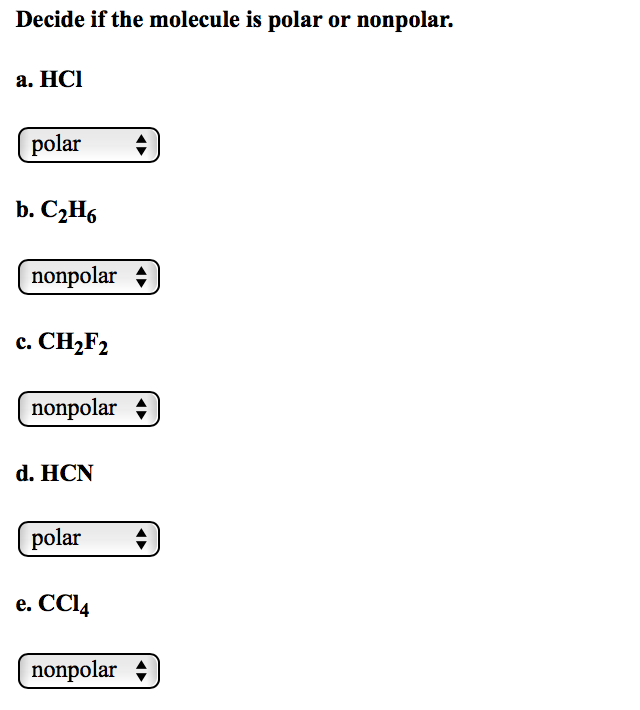
Solved Label the bond formed between I and each of the
Learn to determine if C2H6 is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structure and then us.
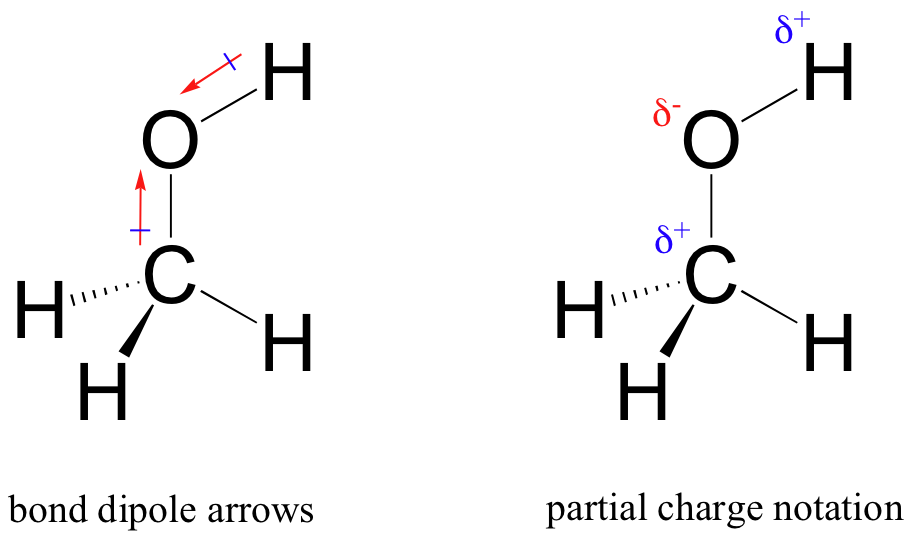
2.3 Interacciones no covalentes LibreTexts Español
Differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. Nonpolar covalent bonds have an equal distribution of electron density between the two nuclei. Polar covalent bonds have an unequal distribution of electron density with the more electronegative atom having greater electron.
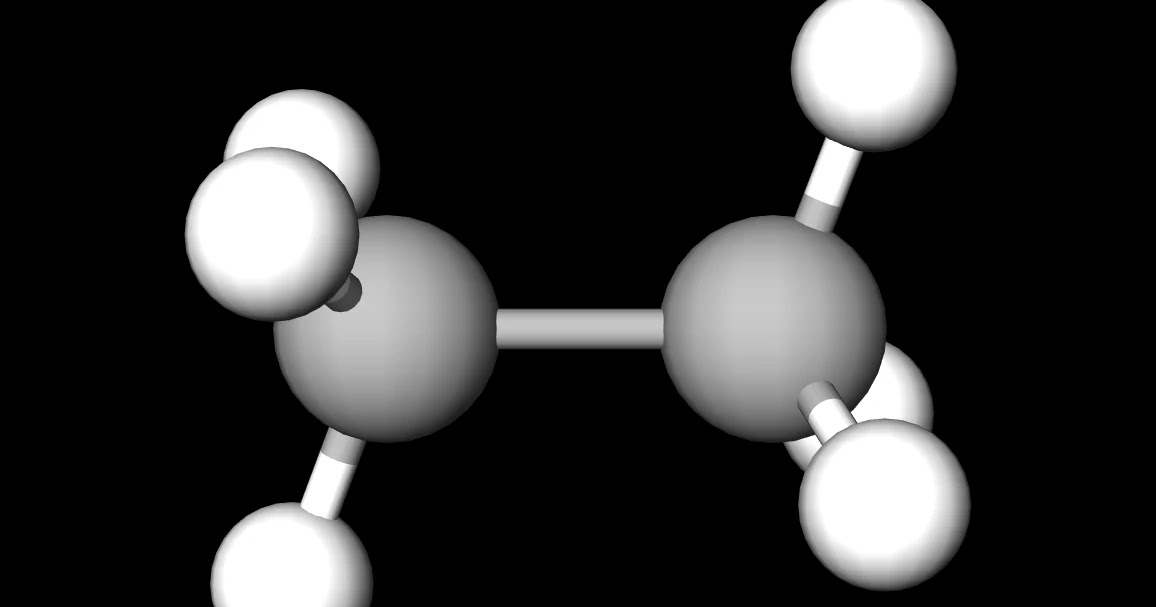
MakeTheBrainHappy Is C2H6 Polar or Nonpolar?
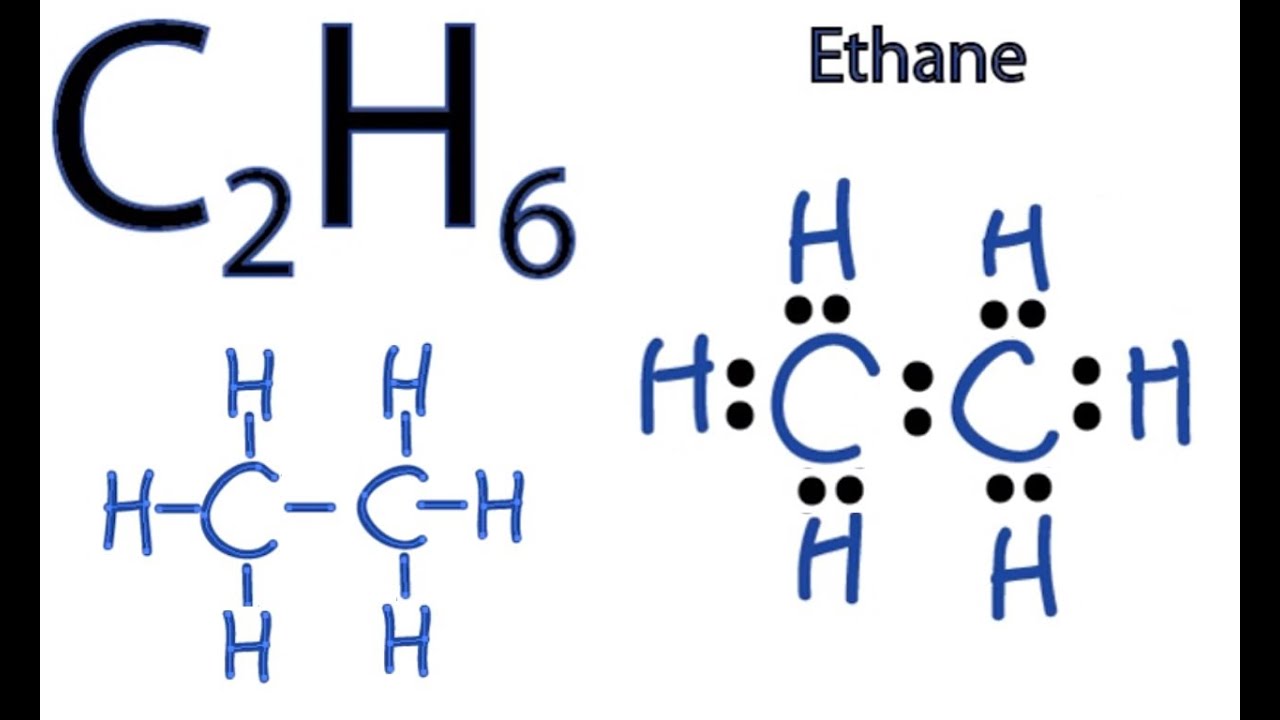
∴ C 2 H 6 has two carbon atoms and six atoms of hydrogen. So, the total valence electrons available for drawing the C 2 H 6 Lewis structure = 2 (4) +6 (1) = 14 valence electrons. 2. Choose the central atom In this second step, usually the least electronegative atom out of all the concerned atoms is chosen as the central atom.
C2h6 Molecule
Read Also: is c2h6 polar? Final Thought. CS2 is a polar or nonpolar molecule, we've examined its structure, bond polarity, and molecular geometry. While the carbon-sulfur bonds in CS2 are polar, the linear molecular arrangement leads to a cancellation of dipole moments, resulting in a nonpolar molecule.

C2h6 Lewis Structure Polar Or Nonpolar Draw Easy
C2H6, known as ethane, is a saturated open-chain hydrocarbon or we can say that it comes under the alkane family. Hydrocarbon is an organic compound, which contains only carbon and hydrogen. Saturated hydrocarbons are those hydrocarbons, which contain carbon-hydrogen and carbon-carbon single bonds.
SOLVED Molecule or Polyatomic Ion 11. NH3; Lewis Structure Data Sketch
Helium is nonpolar and by far the lightest, so it should have the lowest boiling point. Argon and N 2 O have very similar molar masses (40 and 44 g/mol, respectively), but N 2 O is polar while Ar is not. Consequently, N 2 O should have a higher boiling point. A C 60 molecule is nonpolar, but its molar mass is 720 g/mol, much greater than that.

Brf3 Polar Or Nonpolar
When the difference is very small or zero, the bond is covalent and nonpolar. When it is large, the bond is polar covalent or ionic. The absolute values of the electronegativity differences between the atoms in the bonds H-H, H-Cl, and Na-Cl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively.

C2h6 polar mı apolar mı?
Ethane or C2H6 is a nonpolar molecule because: There is significantly less difference in the electronegativities of Hydrogen and Carbon forming bonds in this structure. There is 0 difference in electronegativity of the Carbon atoms forming bonds with each other.